Media & Events
Read the latest news, updates and more.


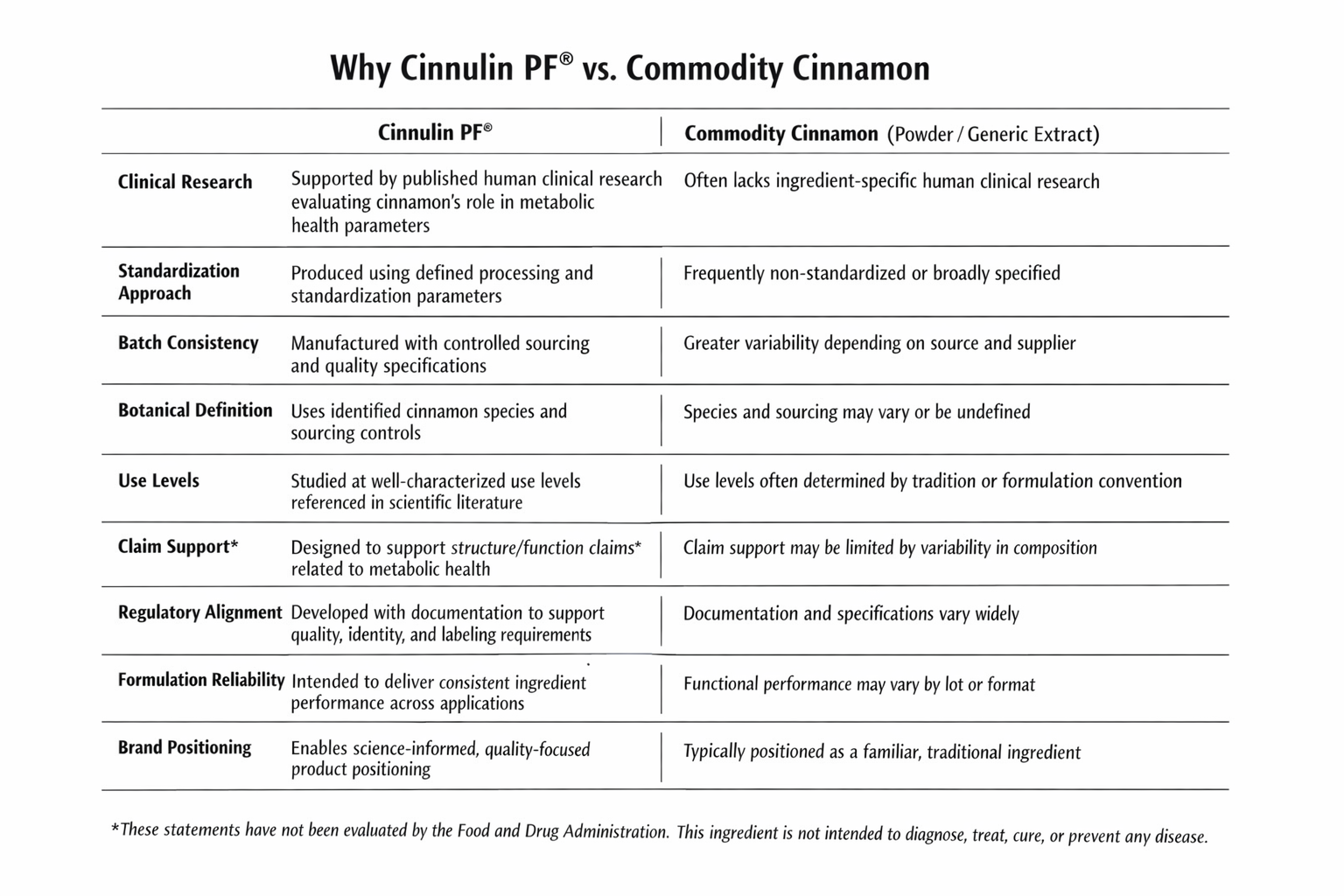
Cinnamon is one of the most widely used botanicals in metabolic health formulations. Yet despite its familiarity, the category remains highly variable—defined by differences in species, sourcing, processing, and compositional consistency.
As regulatory expectations increase and brands place greater emphasis on evidence-based positioning, formulators are reassessing how cinnamon ingredients are selected, specified, and supported within finished products.
Moving Beyond Tradition-Only Ingredient Selection
Many cinnamon ingredients currently used in dietary supplements are selected based on historical use rather than ingredient-specific scientific evaluation. These materials may vary significantly in polyphenol composition, botanical definition, and batch consistency—factors that can influence formulation predictability and documentation readiness.
In contrast, some cinnamon extracts have been developed with greater emphasis on characterization, standardization, and evaluation within human research settings. One such ingredient, Cinnulin PF®, was recently recognized as a Top Pick in ConsumerLab’s 2025 cinnamon supplement review, reflecting its distinct positioning within a highly commoditized category.
Why Clinical Research Matters to Formulators
For formulation teams, ingredient selection increasingly involves more than supply availability or historical precedent. Considerations now commonly include:
- Ingredient identity and specification clarity
- Reproducibility of composition
- Availability of published research
- Alignment with structure/function claim frameworks*
- Quality documentation to support regulatory review
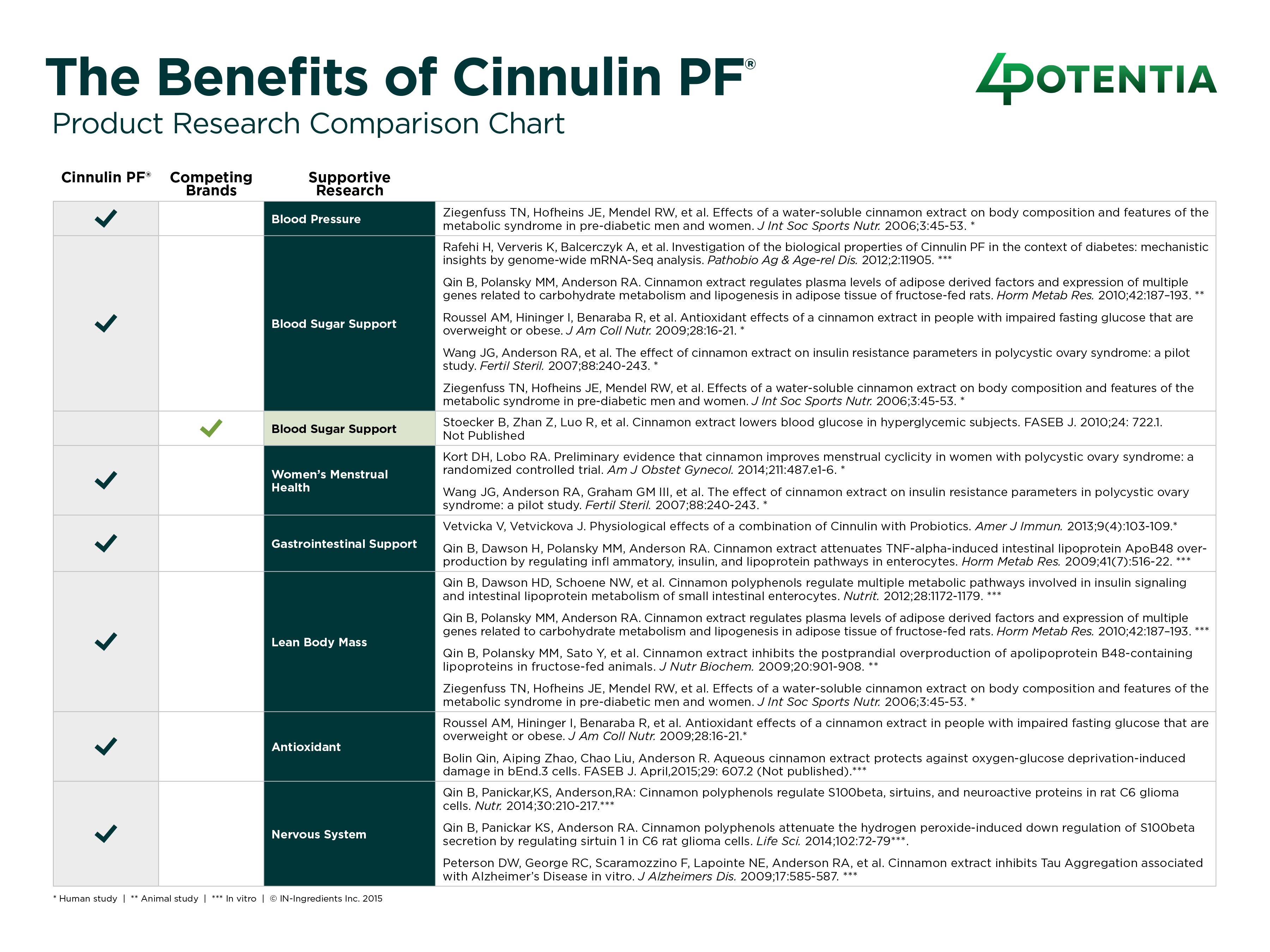
Cinnulin PF® is supported by published human clinical research evaluating cinnamon’s role in metabolic health parameters, including support for healthy glucose metabolism and insulin function*. This research-informed approach provides formulators with additional context when developing products intended for evidence-based positioning.
Ingredient Differentiation in a Commodity-Heavy Category
Cinnamon remains one of the most familiar—but least consistently defined—botanical ingredients on the market. Variability in species selection, processing methods, and standardization can introduce formulation challenges, particularly for brands seeking consistency across production runs or long-term product lines.
Cinnulin PF® was developed with defined sourcing, processing controls, and compositional specifications intended to support batch-to-batch consistency and predictable formulation behavior.
Formulation and Regulatory Considerations for Modern Brands
As consumer expectations shift toward transparency and regulatory oversight increases, ingredient documentation and compositional clarity have become central to product development decisions.
Cinnulin PF® is supported by technical documentation intended to assist with quality review, formulation planning, and labeling considerations. The ingredient is already utilized in commercially available dietary supplements, reflecting its compatibility with real-world manufacturing and compliance environments.
For formulators, this approach supports:
- Clean-label formulation strategies
- Science-informed product narratives
- Consistent ingredient specifications over time
A Broader Shift in Botanical Formulation
Recognition from third-party evaluators such as ConsumerLab reflects a broader industry movement toward botanicals that are more clearly defined, consistently produced, and supported by published research.
For brands developing next-generation metabolic health and wellness products, ingredient selection is increasingly about balancing tradition with documentation, consistency, and long-term formulation reliability. In this context, clinically studied cinnamon extracts like Cinnulin PF® represent an evolution in how familiar botanicals are evaluated and applied.
*These statements have not been evaluated by the Food and Drug Administration. This ingredient is not intended to diagnose, treat, cure, or prevent any disease.

4POTENTIA is excited to be back at SupplySide West in 2025! Catch us on October 29th and 30th at the Mandalay Bay Convention Center in Las Vegas.
Explore what’s new in our ingredient lineup, and see how 4POTENTIA is shaping the future of health and wellness.
Let’s build something incredible—together.
We can’t wait to see you there! 💡
Please stop by our booth #5089.

4POTENTIA Named Cover Story in Food Business Review — Pioneering a New Era in Natural Health Ingredients
We’re proud to share a major milestone for 4POTENTIA: we are the April 2025 cover story in Food Business Review, recognized for pioneering a new era in natural health ingredients and named Health Ingredients Product of the Year 2025.
The feature highlights 4POTENTIA’s evolution, leadership vision, and unwavering commitment to clinically validated, proprietary ingredients that help brand partners compete — and consumers trust what they’re taking.
From Legacy Ingredient Supplier to Innovation Platform
Founded more than 25 years ago as IN Ingredients, the company underwent a strategic transformation in 2024, reemerging as 4POTENTIA with a renewed mission: to bridge nature and science through differentiated, evidence-based ingredients
Under the leadership of CEO Jade Beutler, whose career spans more than three decades in the natural products industry, 4POTENTIA expanded its infrastructure, intellectual property strategy, and partner-first approach — signaling far more than a rebrand. It marked a declaration of intent.
“Our ingredients are unique, novel, patented, or proprietary. This gives our brand partners a competitive edge and ensures consumers receive products they can trust.”
Built for Brands, Backed by Science
4POTENTIA operates exclusively in the B2B ingredient space, supplying branded raw materials to leading dietary supplement companies including Bluebonnet® and Orthomolecular Nutrition®
Unlike commodity ingredients, every 4POTENTIA innovation is supported by rigorous scientific validation. Today, the company’s portfolio includes:
• 26 issued patents
• 12 human clinical studies
• Extensive proprietary and third-party research packages designed to support formulation, substantiation, and commercialization
This science-first model reflects a deliberate shift away from “faith-based” supplementation toward substantiated wellness support that aligns with modern regulatory expectations.
Cinnulin PF®: A Category-Defining Ingredient
The cover story spotlights Cinnulin PF®, 4POTENTIA’s flagship cinnamon extract and the leading branded cinnamon ingredient on the market. Standardized for Type-A polymers, Cinnulin PF® supports healthy glucose metabolism, insulin function, and body composition
Its impact is reinforced by adoption from major brands such as Sam’s Club Member’s Mark® and Doctor’s Best®, and by recent recognition from ConsumerLab® as a Top Pick in cinnamon supplements — further validating its market leadership.
Looking Ahead: BIO-DiMaX™ and the Next Wave of Innovation
The article also offers a preview of what’s next for 4POTENTIA, including the upcoming launch of BIO-DiMaX™, a breakthrough DIM ingredient engineered to solve long-standing bioavailability challenges.
With a patented delivery system shown to be 58× more bioavailable than conventional DIM, BIO-DiMaX™ represents a new standard in hormonal health formulation — delivering results at lower doses with improved tolerability
“We’re not just improving bioavailability; we’re setting a new standard for what’s possible in hormonal health supplements.”
More Than Ingredients — A True Partner Model
What ultimately distinguishes 4POTENTIA is its collaborative approach. Beyond supplying ingredients, the company equips partners with evidence packages, IP protection, and strategic support to help products succeed in a crowded marketplace.
As highlighted in Food Business Review, this philosophy continues to drive growth, innovation, and long-term brand partnerships.
Read the Full Cover Story
Read the full April 2025 Food Business Review cover story featuring 4POTENTIA
We’re honored by this recognition and excited for what’s ahead as we continue to pioneer what’s next in natural health ingredients.

4POTENTIA will be at SupplySide West this year! Join us on October 30th and 31st at the Mandalay Bay Conventions Center in Las Vegas.
Come visit us at booth #3477 to meet our team, learn about our innovative solutions, and discover how 4POTENTIA can help power your potential.💡
Let's connect, collaborate, and explore new opportunities together!
Looking forward to seeing you there!

IN-Ingredients celebrates their 25th Anniversary with a fresh new look and feel becoming 4POTENTIA with the tagline “A Force of Nature.”
Marking this important milestone is the addition of top talent to begin this exciting new chapter. Veteran industry executive Jade Beutler has accepted the position of CEO. He is joined by another industry veteran John Mai, Chief Business Officer.
4POTENTIA vows to bring new, novel, proprietary and patented branded raw materials to market to enhance health, happiness and wellbeing.
Original IN-Ingredients founders Tim and Gus Romero will be staying on as strategic advisors during the transition to 4POTENTIA.
Read the press release here.
